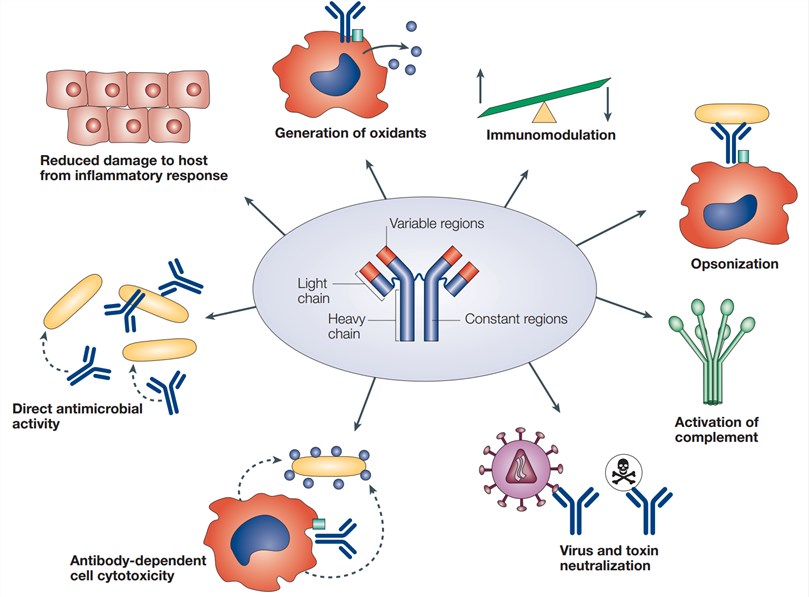
PK/PD/ADA Antibodies and Assays Because all biological therapeutics can induce an immune response that ranges from benign to severe adverse effects, evaluating unwanted immunogenicity is a critical step in earlystage researchrequiring both a wellconceived strategy and fitforpurpose assays for antibody detection and characterizationDefinition Possible cause Potential assay impact; Blood samples for PK analysis were collected during the inpatient phase, predose and up to 96 hours postdose, and during the ambulatory visits in each period Pegfilgrastim concentrations in serum were determined using a standard quantitative enzymelinked immunosorbent assay (ELISA) technique
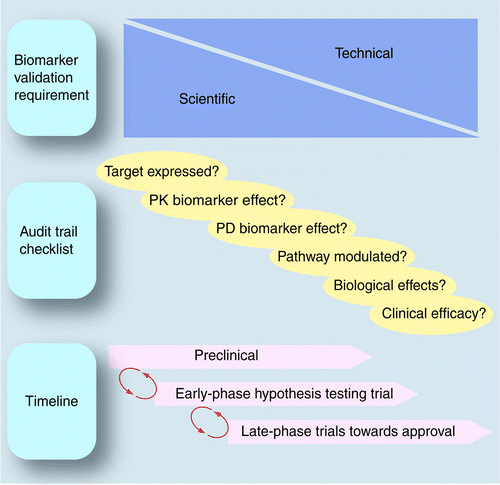
Use Of Pharmacokinetic Pharmacodynamic Biomarkers To Support Rational Cancer Drug Development Biomarkers In Medicine
Pk assays definition
Pk assays definition-Into the assay's quantitative range and multiplying the measured concentration by the dilution factor –Particularly relevant to LBA assays where samples of high concentration may require significant dilution to achieve the working range of the assay –Hook effect is typically assessed in the same experiment Commonly, PK is illustrated by a plasma concentrationtime curve, but the pharmacokinetic profile of a compound includes all the factors that shape it, including the basic processes of absorption, distribution, metabolism, and excretion (ADME) A drug's ADME properties (along with other components of the pharmacokinetic profile) can make it



Submodel Assay Definition
John L Allinson, FIBMS, believes despite the increased use of biomarkers, it appears that many researchers are still continuing to use the FDA guidance document for validation even though it only critically addresses the validation of assays to support PK evaluation, and also has a limited scope described within the document in terms of studies where it should be usedIntroduction Pharmacokinetics (PK) is the analysis and description of the disposition of a drug in the body, encompassing development of the mathematical description of all dispositional processes in the body, defined as ADME – absorption, distribution, metabolism, and eliminationAssay recovery is assessed by comparing observed vs expected values based on nonspiked and/or neat (undiluted) samples Interpreting linearityofdilution results Poor linearity of dilution indicates that the natural sample matrix, the sample diluent and/or standard diluent affect analyte detectability differently
PK/PD is listed in the World's largest and most authoritative dictionary database of abbreviations and acronyms PK/PD What does PK/PD stand for?33 PK/PD relationship Validation of the analytical assay should comprise two distinct phases the prestudy phase and the withinstudy phase In the prestudy phase, the compliance of the assay with respect to (1) stability of the analyteAn assay is an investigative procedure in laboratory medicine, mining, pharmacology, environmental biology and molecular biology for qualitatively assessing or quantitatively measuring the presence, amount, or functional activity of a target entity The analyte can be a drug, biochemical substance, chemical element or compound, or cell in an organism or organic sample The measured entity is
Regarding lottolot bridging for PK assays, consensus was that bridging comparison across the entire assay range should be performed For ADA assays, it is necessary to evaluate the assay performance around the cut point, sensitivity, and drug toleranceADA positives go to NAb assays • ligandbinding assays that measure neutralization of binding • cellbased assays that measure the neutralization of a biological effect of the drug • Cellbased assays, in contrast to ligandbinding assays, are considered to provide a readout that is most representative of the biological effect elicitedPk/pd assays are one of the first steps in determining the potential utility of a specific compound for antimicrobial activity Efficacy and safety at specific dosages must be determined and potential side effects need to be identified Over the years many assays have been




Hit To Lead And Lead To Candidate Optimisation Using Multi Parametric Principles Drug Discovery World Ddw



Www Pharmasug Org Proceedings 18 Pharmasug 18 12 Pdf
Assay Progression Scheme SHSY5Y DRC LQT1minK, Ltype Ca, hERG DRC KCNQ2/Q3, KCNQ3/Q5 DRC (flux) In vitro selectivity KCNQ2/Q3, KCNQ3/Q5 DRC (flux) In vitro ADME CYP, PPB, LM, Sol Early assessment of in vitro ADME In vivo activity Rat MES screen 10mg/kg w/ plasma/brain levels and estimate of metabolites KCNQ2/3, hERG EP and early in vivo PKFrontiers Events is a rapidly growing calendar management system dedicated to the scheduling of academic events This includes announcements and invitations, participant listings and search functionality, abstract handling and publication, related events and postevent exchanges Whether an organizer or participant, make your event a Frontiers Event!Bioanalytical analysis is a fundamental tool for the pharmacokineticist The results of a bioanalysis are the source data for all pharmacokinetic work Thus a clear understanding of the methodologies and challenges associated with the bioanalytical analysis science is of great benefit to a pharmacokineticist




Pharmacodynamics Wikipedia




Overview Of The Main Tasks Of Dmpk Support During The Different Phases Download Scientific Diagram
Assay Optimization (prevalidation) Assay optimization and prevalidation are experiments that determine how a range of matrix and sample elements, as well as assay conditions, effect assay parameters and assay performance These data, along with scientific judgment, set the acceptance criteria for the assay validation The ability to design, construct and run assays that are specific, sensitive and robust is crucial in all areas of biomedical research By way of example, in basic research applications one may need to quantify cellular levels of a particular protein, determine the levels of a metabolite in serum or urine or, perhaps, compare the catalytic activity of an enzyme in normalAssay formats can include but are not limited to, sandwich, competition, direct or indirect binding, inhibition, and solid phase or solution phase assays A typical batch is defined as a set of standard calibrators, validation samples and/or QC samples, and/or study samples



Assay Validation Biomarker Assay Validations A Time For Change




Pharmacokinetics Pharmacodynamics Safety And Immunogenicity Of Pelmeg A Pegfilgrastim Biosimilar In Healthy Subjects Roth 19 Pharmacology Research Amp Perspectives Wiley Online Library
Excess assays, like the common twosite immunometric assay (IMA), there is an increased chance of a crossreactant forming a bridge between the two antibodies Conformational changes to antigens can be induced by antibodies which may alter the specificity of antibodies For these reasons there may be a higher prevalence of unpredictable crossRheumatoid factor (RF) Human antibodies most commonly binding to the Fc fragment of human IgG, other specificities have been described RFs are most typically IgM isotype immunoglobulins Disease type or unknown PK—often leads to over recovery ADA—false positive score Heterophilic antibodyThe term pharmacokinetics (PK) refers to the study of How fast and how completely the drug is absorbed into the body (from the stomach and intestines if it's an oral drug) How the drug becomes distributed through the various body tissues and fluids, called body compartments (blood, muscle, fatty tissue, cerebrospinal fluid, and so on)
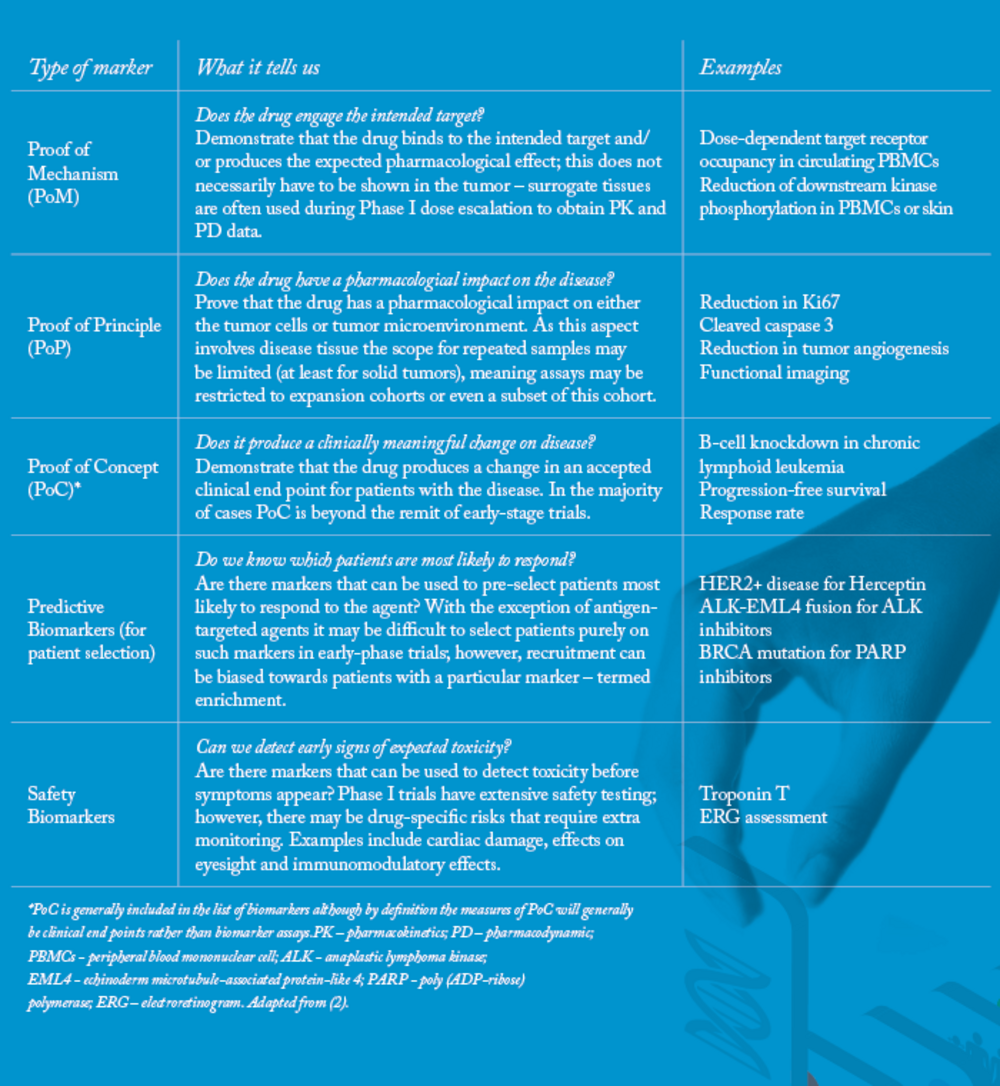



No Biomarker No Trial




New Fda Guidance On Developing Validating Ada Assays For Ada Antibody Detection
While PK assays follow multiple formats, the standard immunogenicity assay relies on a bridging format wherein the drug is used for both capture and detection of the ADA The advantage of the bridging format lies in the fact that it only uses the variable regions of the ADA positive control, thus allowing the use of surrogate positive controlsAssays (CCs) and ligand binding assays (LBAs) that quantitatively determine the levels of drugs, their metabolites, therapeutic proteins, and biomarkers in biological matrices such asAssay Validation & Performance Reports Biologics only • Immunogenicity • Comparability Clinical Pharmacology • FirstinHuman • SAD and MAD PK Studies • Healthy vs Patient population



Www Mdpi Com 1999 4923 13 3 422 Pdf



Www Lexjansen Com Phuse Us 18 Dh Dh05 Pdf
Looking for online definition of PK/PD or what PK/PD stands for?Pharmacokinetic / Toxicokinetic (PK/TK) Analysis Pharmacokinetics (PK) is the determination of the extent of Absorption, Distribution, Metabolism and Excretion (ADME) of a small molecule compounds, peptides, and biologics/large molecules KCAS can support your pharmacokinetic needs with noncompartimental analyses and also working with What is Quantification Anyway?




Pharmacokinetic Assay An Overview Sciencedirect Topics




Improving The Accuracy Of Predicted Human Pharmacokinetics Lessons Learned From The Astrazeneca Drug Pipeline Over Two Decades Trends In Pharmacological Sciences
PK assay bioanalytical testing methods are used to determine concentration time profiles of the drug and metabolites in biological sample fluids, providing information necessary for PK analysis PK assays are a vital component of the drug development process, and the data derived is used to help select dosage for preclinical and clinical studiesCLIA applicability is determined using the regulatory definition of "laboratory" quoted above Specifically, CLIA applies when (1) patientspecific results are reported from the laboratory to system, assay or examination does not appear on the lists of tests in the Federal Register notices,Rheumatoid factor (RF) Human antibodies most commonly binding to the Fc fragment of human IgG, other specificities have been described RFs are most typically IgM isotype immunoglobulins Disease type or unknown PK—often leads to over recovery ADA—false positive score Heterophilic antibody




Approaching Sites Of Action Of Drugs In Clinical Pharmacology New Analytical Options And Their Challenges Longuespee 21 British Journal Of Clinical Pharmacology Wiley Online Library
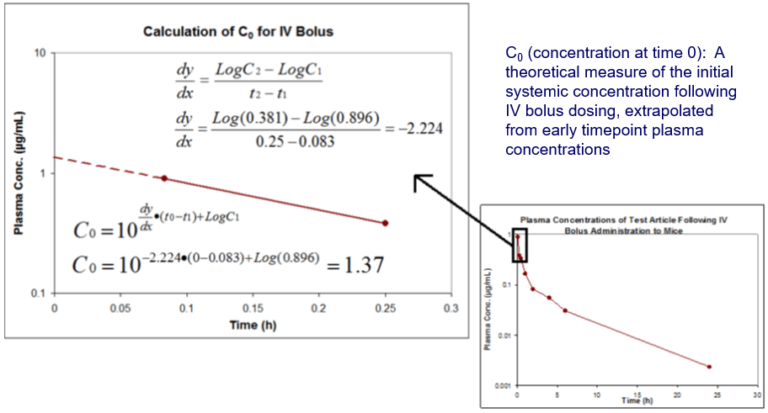



Pharmacokinetics Pk Pharmacodynamics Pd Pk Pd Northeast Biolab
The result of PK biomarker assessment studies at Creative Biolabs is accurate and reliable to ensure highquality PK assay services for our clients all over the world In addition to PK biomarker assessment services, Creative Biolabs also offers other in vivo PK study services, including Parallel/Crossover Study Cassette Dosing Studies PK and PD results from animal studies can be incorporated into PK/PD models and used to guide firstinhuman dose selection Then an iterative PK/PD modeling and simulation process begins to refine the dose from early to late phase of clinical development Reliable PK and PD data is a prerequisite for successful PK/PD modeling and simulationLIGAND BINDING ASSAYS (PK) studies and of clinical trials, including 97 comparative bioavailability/(BA/BE) studiesbioequivalence , are used to make regulatory 98 decisions regarding the safety and efficacy of drug products It is therefore critical that the




Pdf Application Of In Silico In Vitro And In Vivo Admet Pk Platforms In Drug Discovery




Improving The Accuracy Of Predicted Human Pharmacokinetics Lessons Learned From The Astrazeneca Drug Pipeline Over Two Decades Trends In Pharmacological Sciences
The role of the comparative PK study in the assessment of biosimilarity is to exclude any relevant PK differences that could indicate presence of structural and/or functional differences that could impact the efficacy, safety or immunogenicity of the product Following are issues that need to be considered in assessment of biosimilarityPAMPA (parallel artificial membrane permeation assay) is an in vitro model of passive diffusion The test compound is added to the donor compartment of a 96 well plate The permeation of compound across an artificial hexadecane membrane is quantified by LCMS/MS after a five hour incubation at room temperaturePK PD assays estimate the safety and efficacy of therapeutics after suitable bioanalysis Pharmacokinetics modeling and simulation help further understand what the body does to a drug, modeling the processes of absorption, distribution, metabolism, and elimination (ADME)




In Vitro And In Vivo Assessment Of Adme And Pk Properties During Lead Selection And Lead Optimization Guidelines Benchmarks And Rules Of Thumb Assay Guidance Manual Ncbi Bookshelf
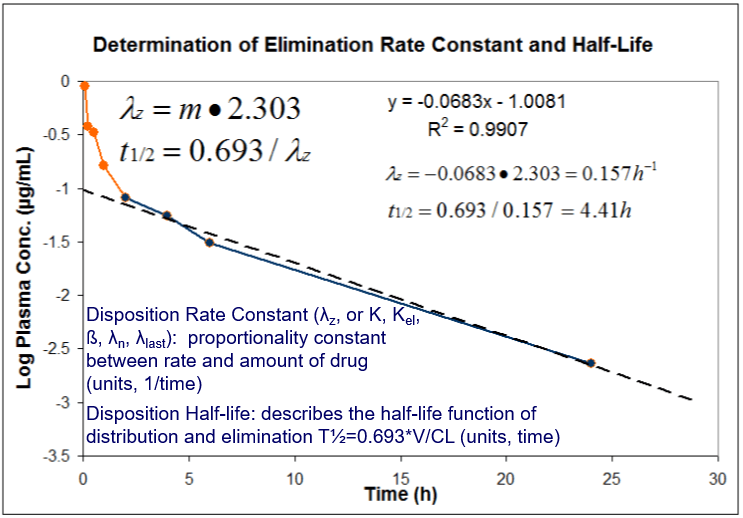



Pharmacokinetics Pk Pharmacodynamics Pd Pk Pd Northeast Biolab
Interassay CV is the variation of the sample measurement on different runs For example, measuring a sample on one plate and the same sample on a separate plate Interassay CV values should ideally be less than 15% Usually the intraassay CV value is lower than the interassay CV because the variation between runs is higher, than on the sameAssay Design The assay is defined during this stage based on knowledge gained through development activities Stage 2 – Assay Qualification During this stage, the assay design is confirmed as being capable of producing reproducible results suitable for the specified purpose Scientifically sound work in progressThe enzyme linked immunosorbent assay (ELISA) is a powerful method for detecting and quantifying a specific protein in a complex mixture Originally described by Engvall and Perlmann (1971), the method enables analysis of protein samples immobilized in microplate wells using specific antibodies ELISAs are typically performed in 96well or 384




Early Drug Development In The Era Of Immuno Oncology Are We Ready To Face The Challenges Annals Of Oncology




Critical Considerations For The Development Of Potency Tests For Therapeutic Applications Of Mesenchymal Stromal Cell Derived Small Extracellular Vesicles Cytotherapy
Definition Possible cause Potential assay impact;Cyprotex is a specialist provider of ADME and PK services and provide a range of in vitro drug metabolism assays Metabolic stability A drug that is rapidly metabolized may require multiple daily dosing or continuous infusion to maintain a concentration in the bloodstream or target organ that is sufficient to elicit a therapeutic effect




Pharmacokinetic Pk Assays Pk Pd Analysis Dmpk Assay



Resources Perkinelmer Com Lab Solutions Resources Docs App Alphalisa Pharmacokinetic Tnfa Pdf




A Pharmacometric Approach To Define Target Site Specific Breakpoints For Bacterial Killing And Resistance Suppression Integrating Microdialysis Time Kill Curves And Heteroresistance Data A Case Study With Moxifloxacin Clinical Microbiology And



Submodel Assay Definition
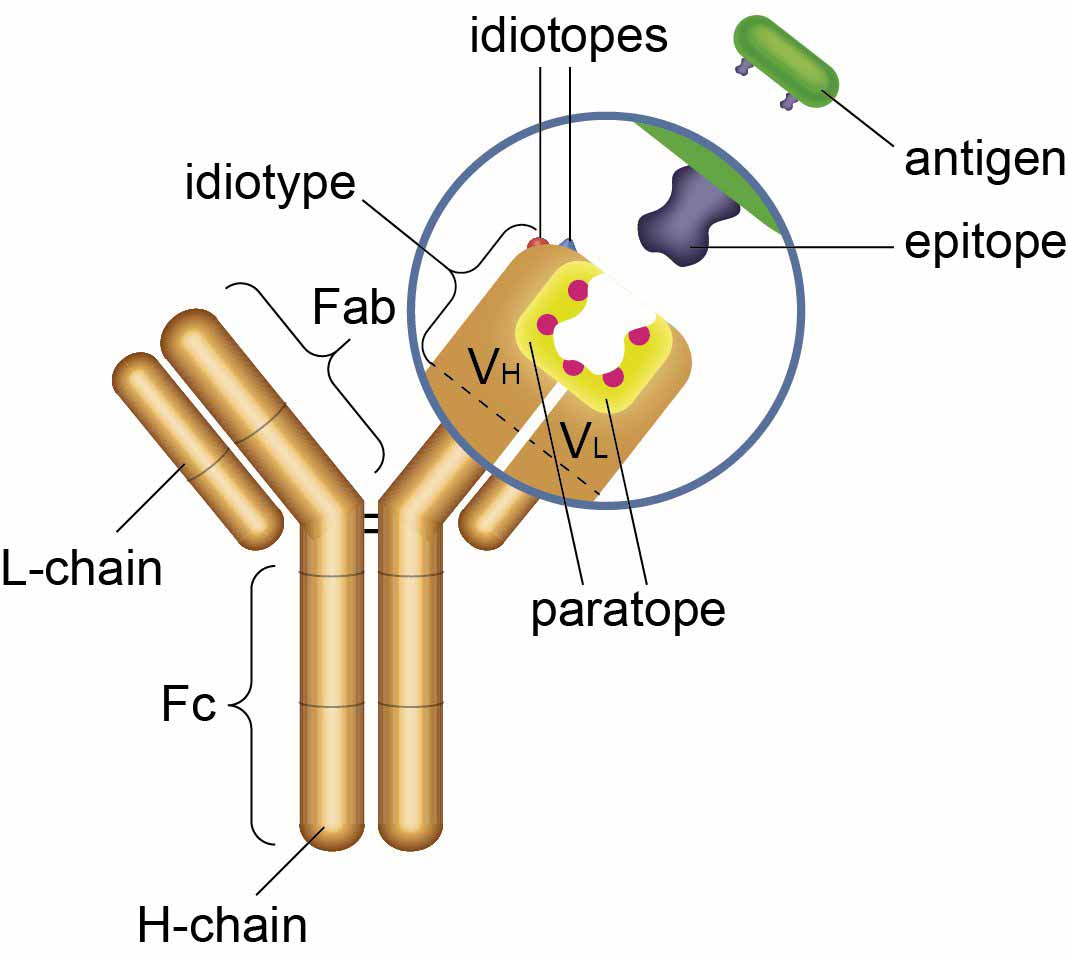



Anti Idiotypic Antibodies Bio Rad



Immunogenicity Testing Regulatory Updates For Immunogenicity Assessment Of Therapeutic Proteins
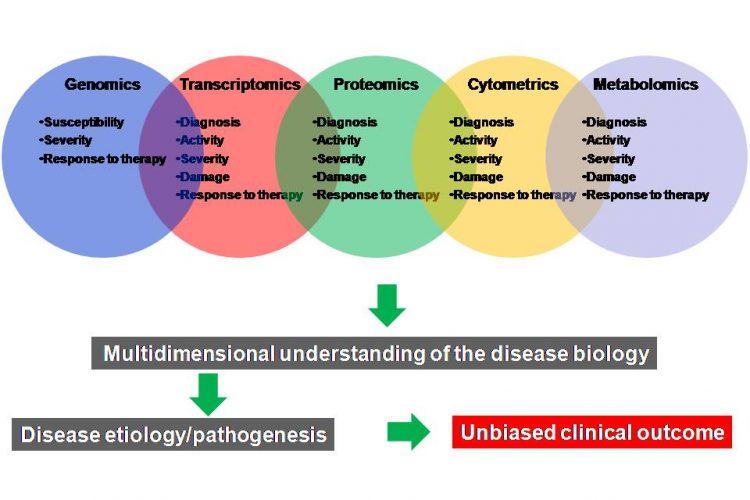



Biomarkers In Drug Discovery And Development




Pdf Pharmacokinetics In Drug Discovery An Exposure Centred Approach To Optimising And Predicting Drug Efficacy And Safety



Http Uu Diva Portal Org Smash Get Diva2 Fulltext02 Pdf




Pediatric Clinical Investigator Training Workshop Ppt Download
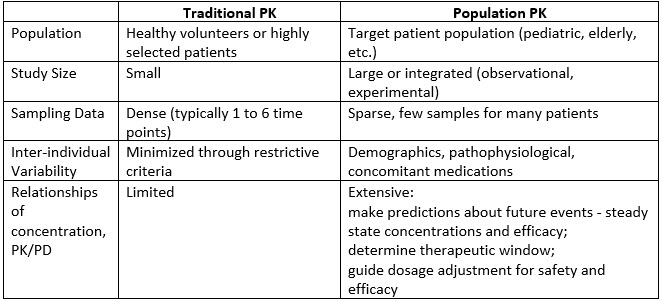



Population Pk Pd Analysis What Why How And When Insights From Our Labs To Yours




Use Of Pharmacokinetic Pharmacodynamic Biomarkers To Support Rational Cancer Drug Development Biomarkers In Medicine



Glaxo Wellcome And Science Global
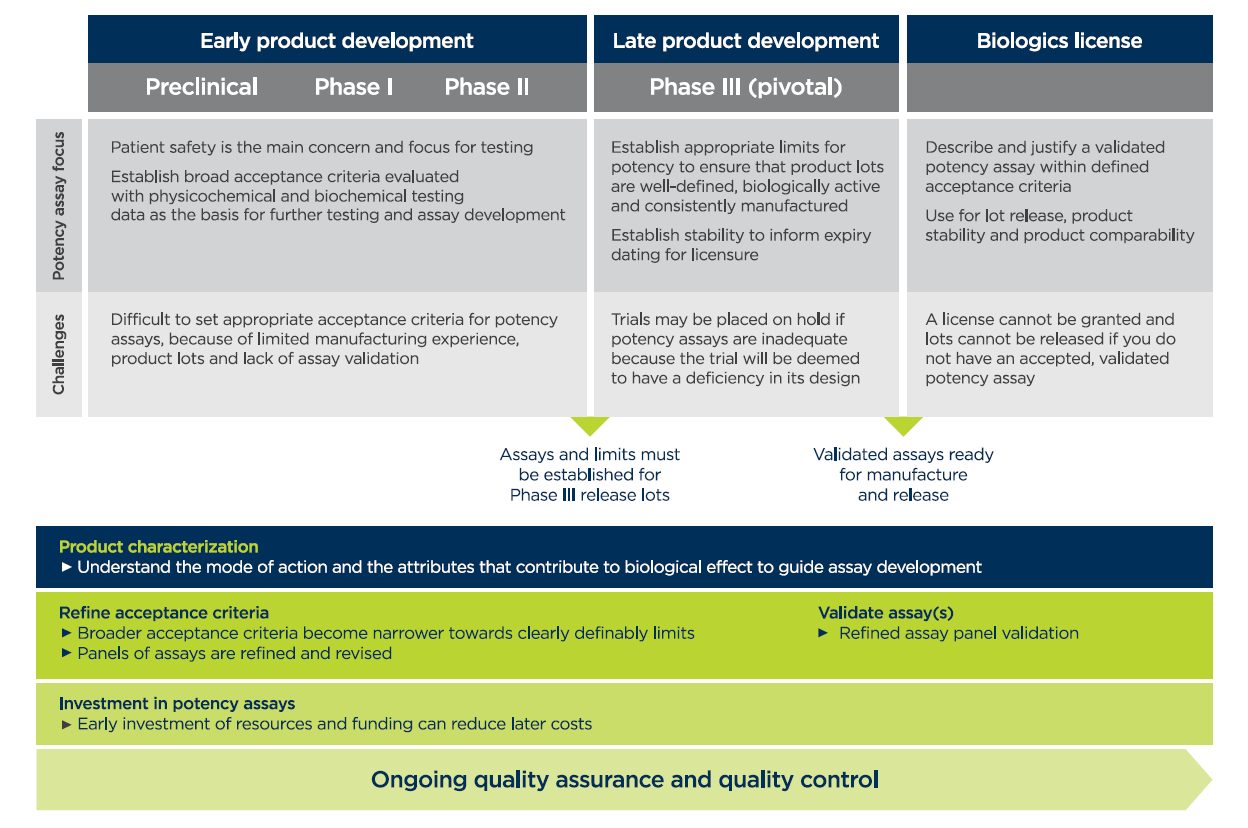



Potency Assays For Atmps Overcoming Challenges On The Path To Commercialization Insights From Our Labs To Yours



Submodel Assay Definition




Managing The Impact Of Immunogenicity In An Era Of Immunotherapy From Bench To Bedside Journal Of Pharmaceutical Sciences



Neutralization Assays Creative Biolabs




Scielo Brazil Non Clinical Studies In The Process Of New Drug Development Part Ii Good Laboratory Practice Metabolism Pharmacokinetics Safety And Dose Translation To Clinical Studies Non Clinical Studies In The



Apps Who Int Iris Bitstream Handle Who Cds Tb 18 6 Eng Pdf




Scientific Challenges For Development Of Biosimilar Monoclonal Antibodies Rafiqul Islam Director Global Bioanalytical Services Celerion Pdf Free Download




Pyruvate Kinase Assay Kit Ab432 Abcam



Www E B F Eu Wp Content Uploads 18 05 n13 S50 B2 Par 2 Clare Kingsley Qbas A Global View On Parallel Pdf



Pharmacokinetics Theory And Application In Drug Discovery And Development Springerlink



Cdn2 Hubspot Net Hubfs 3 Gyros Marketing material Pis Pis Gyrolab Bioaffy Cds Pdf




Discovery And Translational Biology Irbm



Www Pharmasug Org Proceedings 18 Pharmasug 18 12 Pdf
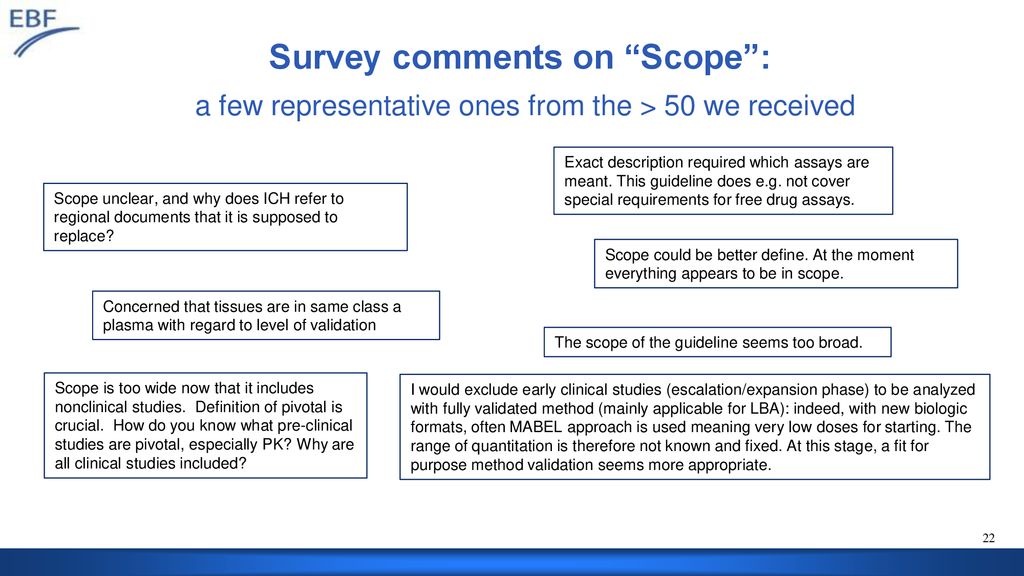



Dv Dmdg Meeting Feedback From Ebf On Ich M10 Ppt Download




Antibody Solutions Pk Pd Ada Antibodies And Assays




Pharmacokinetics Wikipedia




17 White Paper On Recent Issues In Bioanalysis A Global Perspective On Immunogenicity Guidelines Biomarker Assay Performance Part 3 Lba Immunogenicity Biomarkers And Pk Assays Bioanalysis




Adme Guide The Definition Of Adme And Pharmacokinetics Pk
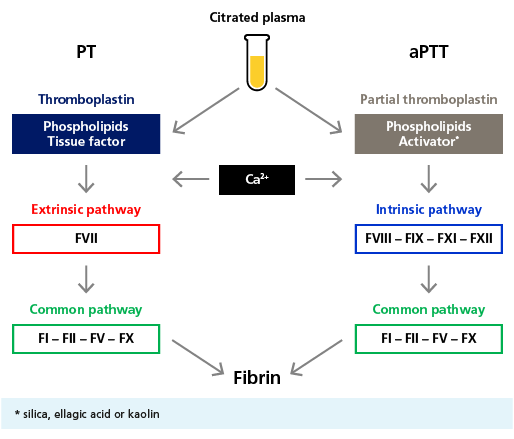



Clot Based Activity Assays




Challenges Of Phase 1 Clinical Trials Evaluating Immune Checkpoint Targeted Antibodies Annals Of Oncology




Quality Requirements For Critical Assay Reagents Used In Bioanalysis Of Therapeutic Proteins What Bioanalysts Should Know About Their Reagents Bioanalysis



Design A New Assay Documentation




Ligand Binding Assay An Overview Sciencedirect Topics




Gyrolab Bioaffy 4000 Cd Extended Immunoassay Sensitivity




0olvnr Yzrlsnm




Functional Assays To Assess The Therapeutic Potential Of Extracellular Vesicles Nguyen Journal Of Extracellular Vesicles Wiley Online Library




Application Of A Plug And Play Immunogenicity Assay In Cynomolgus Monkey Serum For Adcs At Early Stages Of Drug Development



Design A New Assay Documentation




Scientific Challenges For Development Of Biosimilar Monoclonal Antibodies Rafiqul Islam Director Global Bioanalytical Services Celerion Pdf Free Download
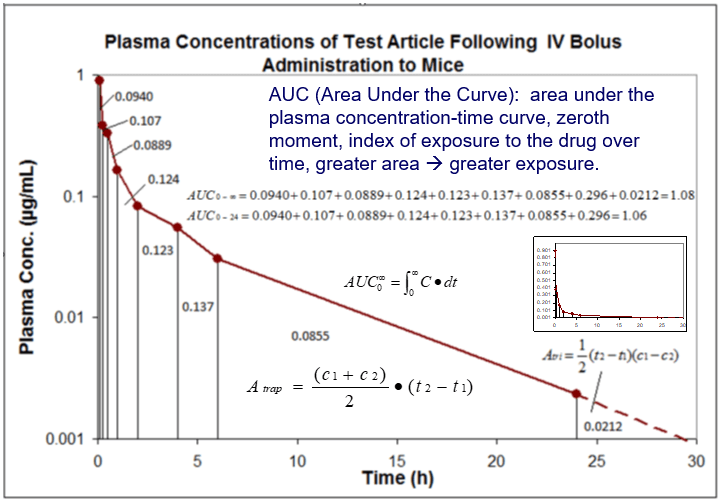



Pharmacokinetics Pk Pharmacodynamics Pd Pk Pd Northeast Biolab




Pharmacokinetics Wikipedia




Ebf Recommendation On Practical Management Of Critical Reagents For Pk Ligand Binding Assays Bioanalysis



Resources Perkinelmer Com Lab Solutions Resources Docs App Alphalisa Pharmacokinetic Tnfa Pdf



Receptor Occupancy Definition Overview Applications




Meso Scale Discovery Msd Pacific Biolabs




Compounds Definition And Work Flows Open Systems Pharmacology




Recommendations For Adaptation And Validation Of Commercial Kits For Biomarker Quantification In Drug Development Bioanalysis



Full Article Bioanalytical Strategy Used In Development Of Pharmacokinetic Pk Methods That Support Biosimilar Programs
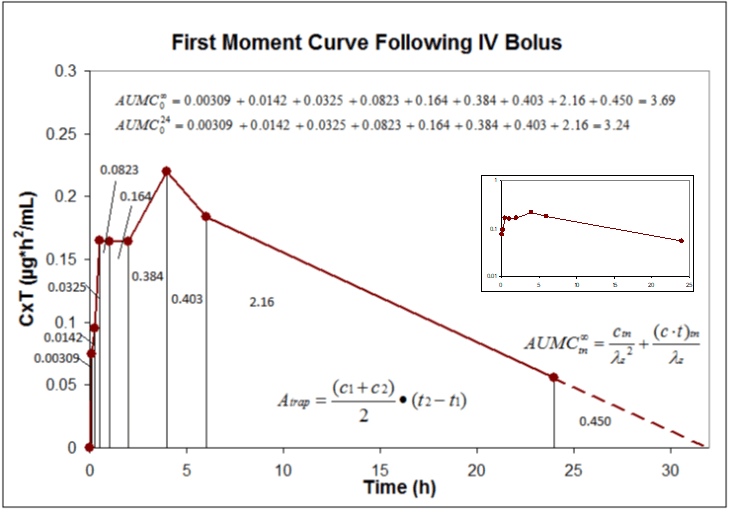



Pharmacokinetics Pk Pharmacodynamics Pd Pk Pd Northeast Biolab



Pdf Parallelism Considerations For The Development Validation And Implementation Of Pk And Biomarker Ligand Binding Assays
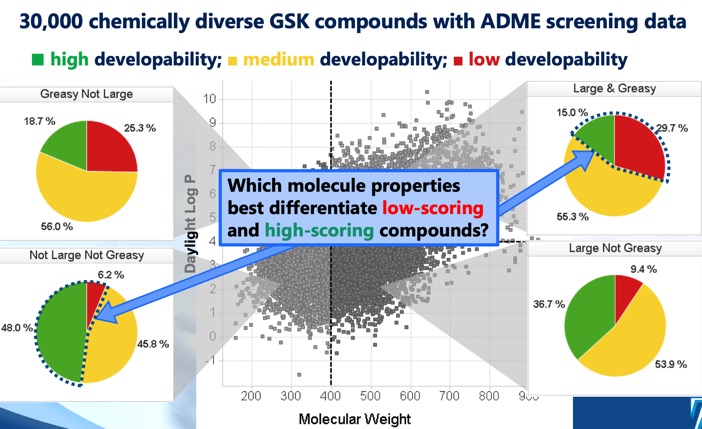



Adme Properties Cambridge Medchem Consulting




Pharmacokinetics In Drug Discovery Ruiz Garcia 08 Journal Of Pharmaceutical Sciences Wiley Online Library
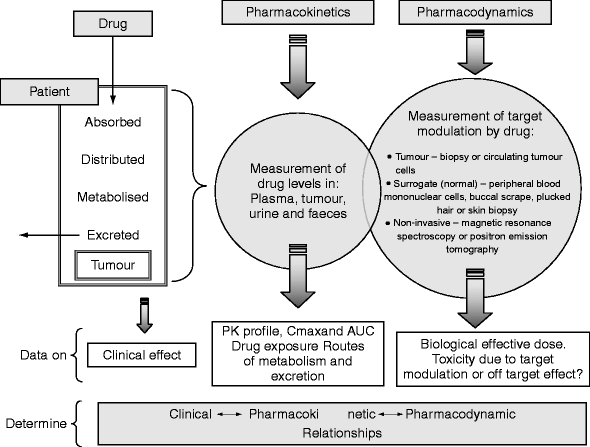



Pharmacokinetics And Pharmacodynamics In Drug Development Springerlink



1



1




Application Of A Plug And Play Immunogenicity Assay In Cynomolgus Monkey Serum For Adcs At Early Stages Of Drug Development




Perspectives Of Bioanalysis In Drug Development Bioanalysis For Service Users




Gyrolab Cds Automated Immunoassays Gyros Protein Technologies




Pharmacokinetic Assay An Overview Sciencedirect Topics




Massively Parallel Reporter Assays Defining Functional Psychiatric Genetic Variants Across Biological Contexts Biological Psychiatry




Pdf Pharmacokinetics In Drug Discovery An Exposure Centred Approach To Optimising And Predicting Drug Efficacy And Safety




Ebf Recommendation On Practical Management Of Critical Reagents For Pk Ligand Binding Assays Bioanalysis
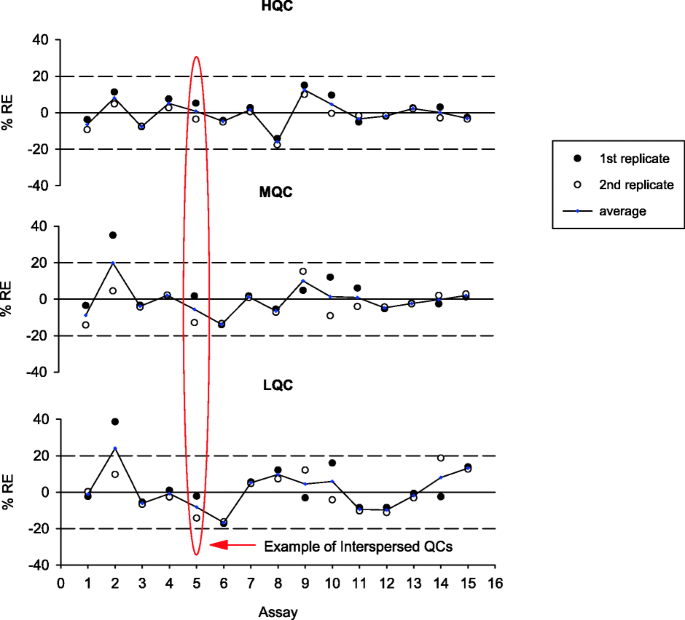



Quality Controls In Ligand Binding Assays Recommendations And Best Practices For Preparation Qualification Maintenance Of Lot To Lot Consistency And Prevention Of Assay Drift Springerlink




Ligand Binding Assay An Overview Sciencedirect Topics




What Is An Anti Idiotypic Antibody




Hit To Lead And Lead To Candidate Optimisation Using Multi Parametric Principles Drug Discovery World Ddw




Pharmacodynamics Wikipedia




Pdf Pharmacokinetics In Drug Discovery An Exposure Centred Approach To Optimising And Predicting Drug Efficacy And Safety




Quality Controls In Ligand Binding Assays Recommendations And Best Practices For Preparation Qualification Maintenance Of Lot To Lot Consistency And Prevention Of Assay Drift Springerlink




Pyruvate Kinase Lactic Dehydrogenase Enzymes From Rabbit Muscle For The Determination Of Adp Buffered Aqueous Glycerol




Chemical Assay Of Drugs And Drug Metabolites Nih Clinical Center




Application Of A Plug And Play Immunogenicity Assay In Cynomolgus Monkey Serum For Adcs At Early Stages Of Drug Development




What Is An Anti Idiotypic Antibody
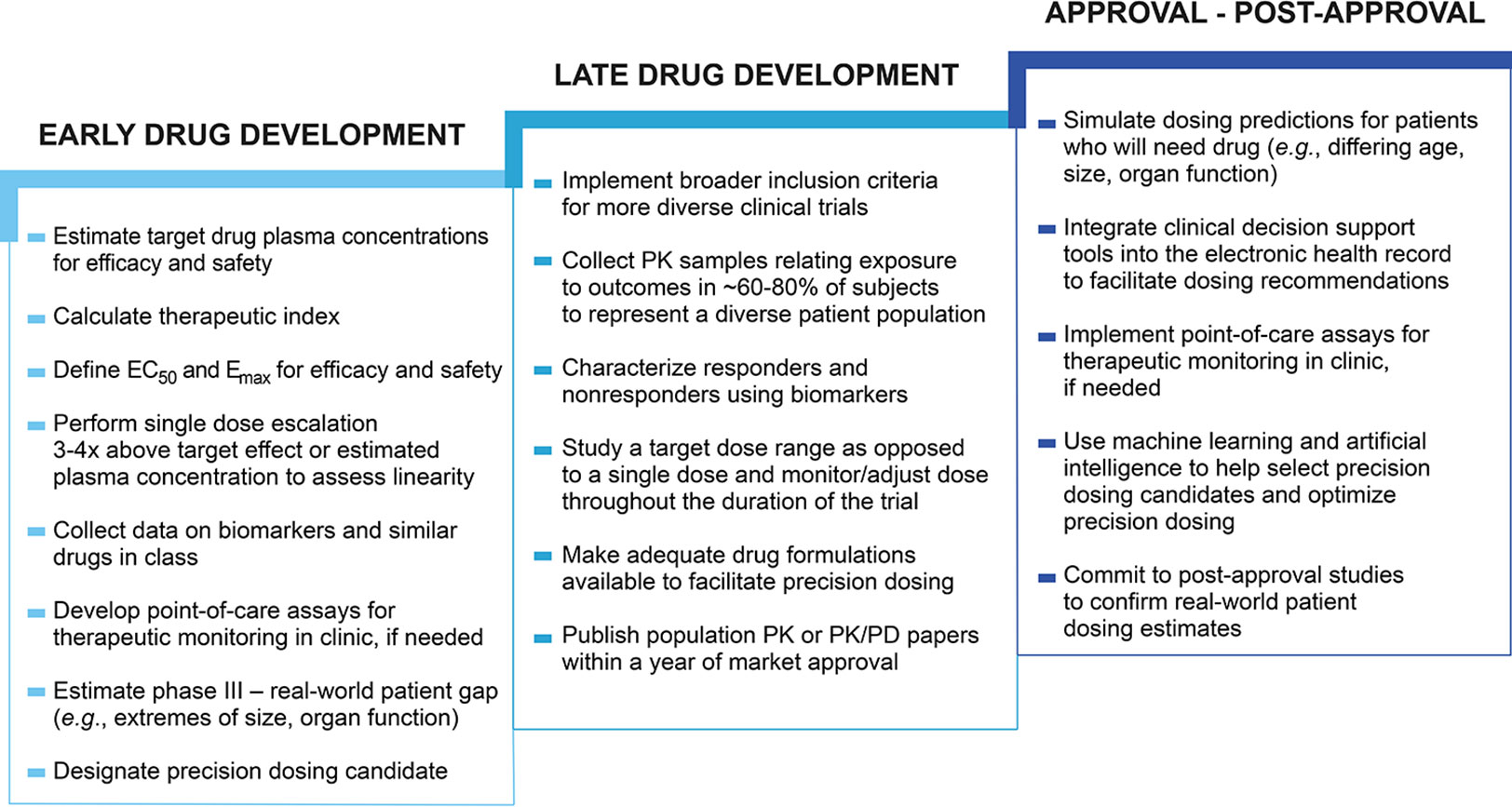



Frontiers Precision Dosing Priority Criteria Drug Disease And Patient Population Variables Pharmacology




Pharmacokinetic Assay An Overview Sciencedirect Topics



1
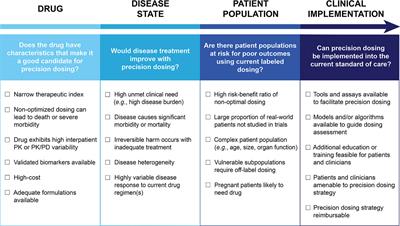



Frontiers Precision Dosing Priority Criteria Drug Disease And Patient Population Variables Pharmacology
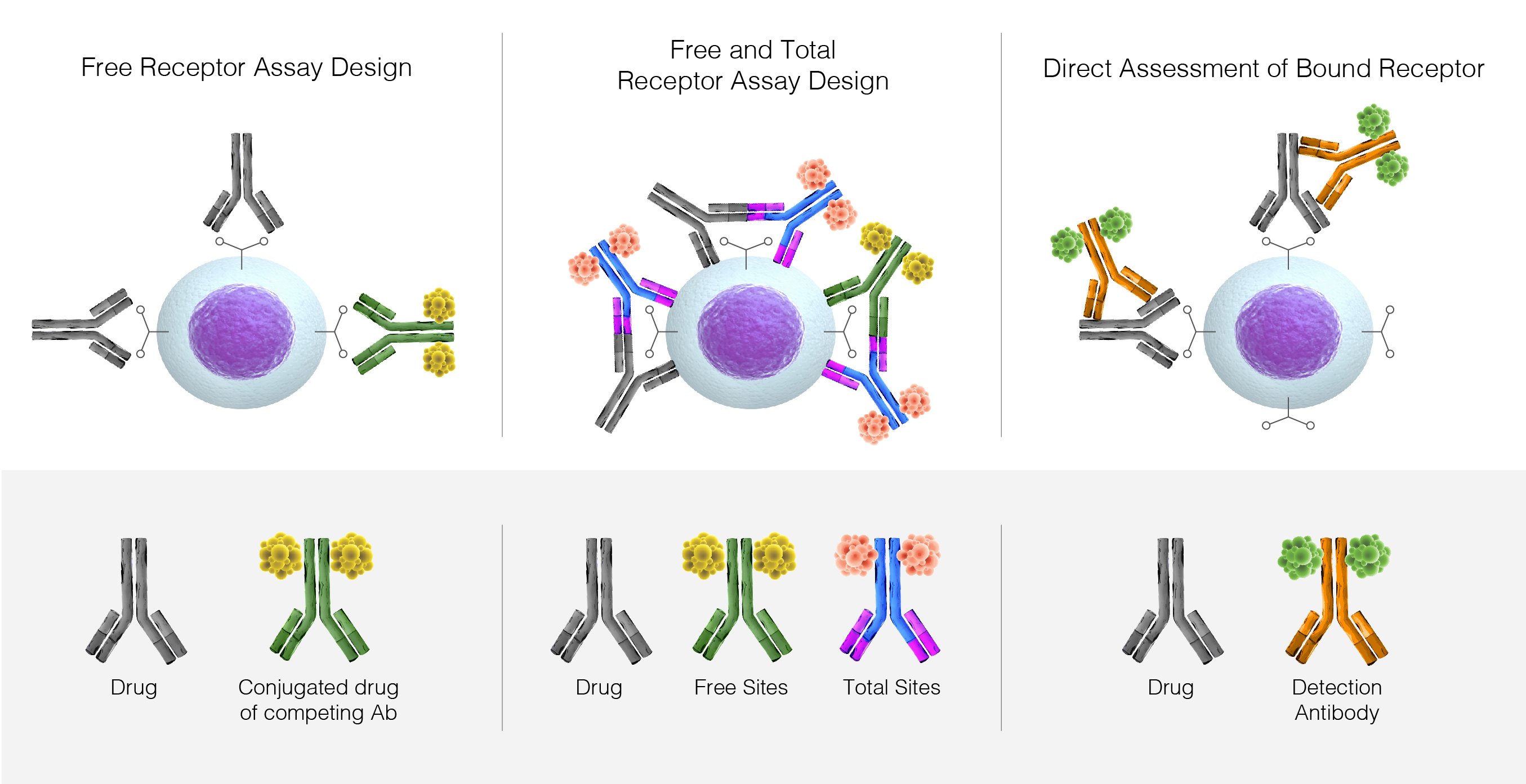



Receptor Occupancy Definition Overview Applications



1




What Is An Anti Idiotypic Antibody
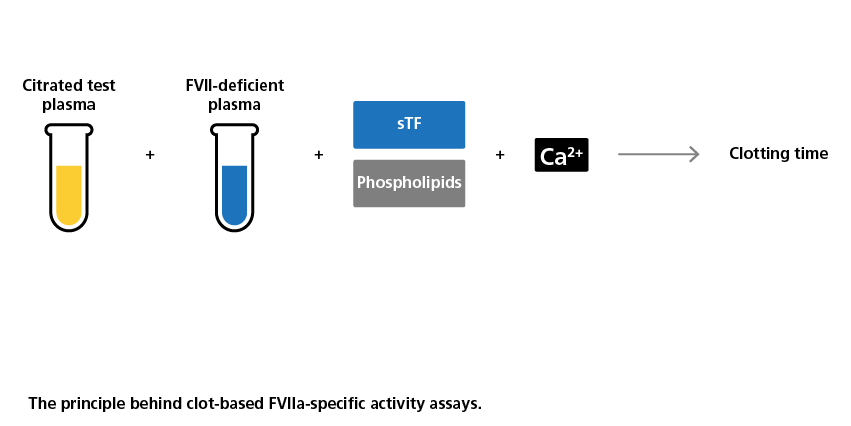



Clot Based Activity Assays



0 件のコメント:
コメントを投稿