PK/PD/ADA Antibodies and Assays Because all biological therapeutics can induce an immune response that ranges from benign to severe adverse effects, evaluating unwanted immunogenicity is a critical step in earlystage researchrequiring both a wellconceived strategy and fitforpurpose assays for antibody detection and characterizationDefinition Possible cause Potential assay impact; Blood samples for PK analysis were collected during the inpatient phase, predose and up to 96 hours postdose, and during the ambulatory visits in each period Pegfilgrastim concentrations in serum were determined using a standard quantitative enzymelinked immunosorbent assay (ELISA) technique
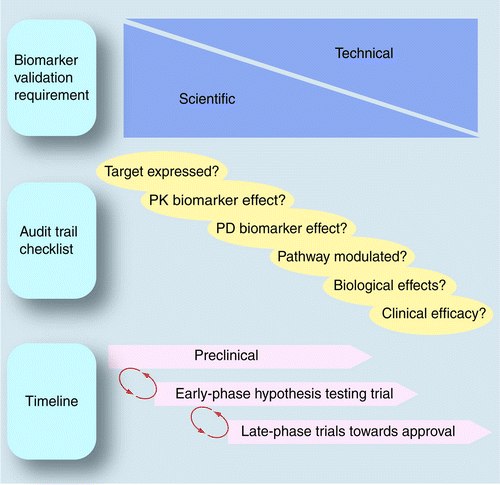
Use Of Pharmacokinetic Pharmacodynamic Biomarkers To Support Rational Cancer Drug Development Biomarkers In Medicine